- Home
- Catalytic Alkane Oxidation
Catalytic Alkane Oxidation:
The fundamental assets in organic synthesis are C-C and C-X (X = O, N, S) bond forming reactions. In contemporary organic synthesis, the activation of inert C-H bonds starting from simple materials, by using a heme and non-heme catalysts and organic oxidative reagents in hydrogen atom transfer (HAT) and radical manner, is an essential challenging task. Metal catalysed oxidation is a key technology for converting the petroleum and other hydrocarbon feedstocks to useful chemicals of a high oxidation state such as epoxides, carbonyl compounds and alcohols. These compounds are producing worldwide in millions of tons and searching the application in all areas of chemical industries. For economic and environmental reasons, oxygen as the primary oxidant in the oxidation processes of bulk chemical industries. The success of metal catalysts to promote the rate of the reaction and the selectivity of partial oxidation process. Transition-metal-catalyzed transformations have emerged a powerful synthetic tool to synthesize C−O and C=O bonds. In the last two decades synthetic chemists have shown significant interest to access a selective hydrocarbon oxidation protocol still represents a major challenge for synthetic chemists. Transition metal coordinated complexes plays a major role in activating the inert C-H bonds in aliphatic systems and bearing the bioactive complex molecules. The development of simple molecules by C−O bonds forming reactions remains the most promising approach in organic synthesis. Wacker oxidation reaction, epoxidation, allylic oxidation, C-H oxidation and alkenes to alcohols and ketones reaction are the best choice for attaining the selectivity and thus for developing ideal synthetic procedures for small molecules. Thus, currently the ultimate aim of the research and industry is improve the development of the selective and active metal catalyst can activate the molecular oxygen at room temperature and the process to fine chemical substrates with high stereoselectivity.
Our ultimate aim is to produce a selective oxidation reactions from explored biologically inspired catalysts. The significance of the catalytic methodologies that provides a selectivites and novel reactivities that could complement those attained with traditional oxidation reactions. The catalytic oxidation process is irrespective of heterogeneous or homogeneous process, classified as i) oxidation based on the mode of monooxygenase, ii) free radical oxidation, iii) substrate coordinated with metal ions, iv) oxidation in mimicking dioxygenase. Among, all oxidation process is a broad interest in synthetic organic chemistry because of the synthetic value of chiral molecules.
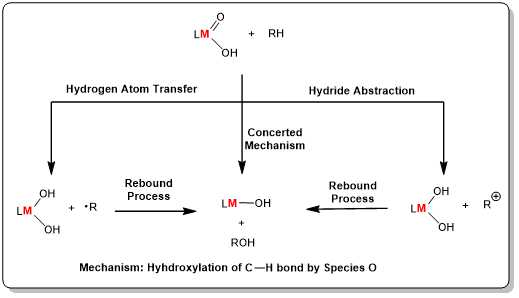
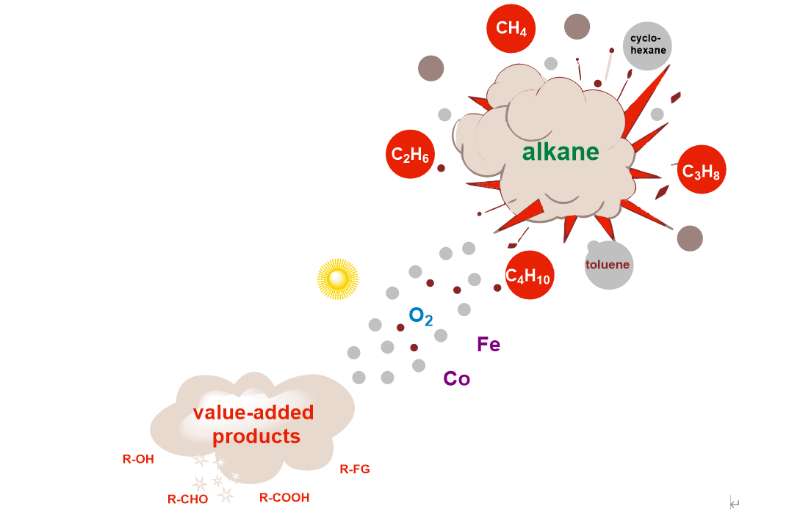
Realizing the direct conversion of low-carbon alkanes under green and economical reaction conditions has been one of the major challenges in the chemical energy sector. However, most of the LCAs exist in gaseous form and are extremely difficult to be liquefied, making them expensive to store and transport. If LCAs can be converted into other liquid products such as alcohols, aldehydes and acids, not only can the transportation problem be solved effectively, but also the high value-added utilization of LCAs can be realized. At the same time, it is also an important direction for us to reveal the mechanism of alkane oxidation through various experimental methods.
Translated with DeepL.com (free version)